News
NAFDAC Alerts Public About Falsified Gold Vision Oxytocin Injection
NAFDAC alerts public about unregistered Gold Vision Oxytocin injection 10IU circulating in Nigeria.
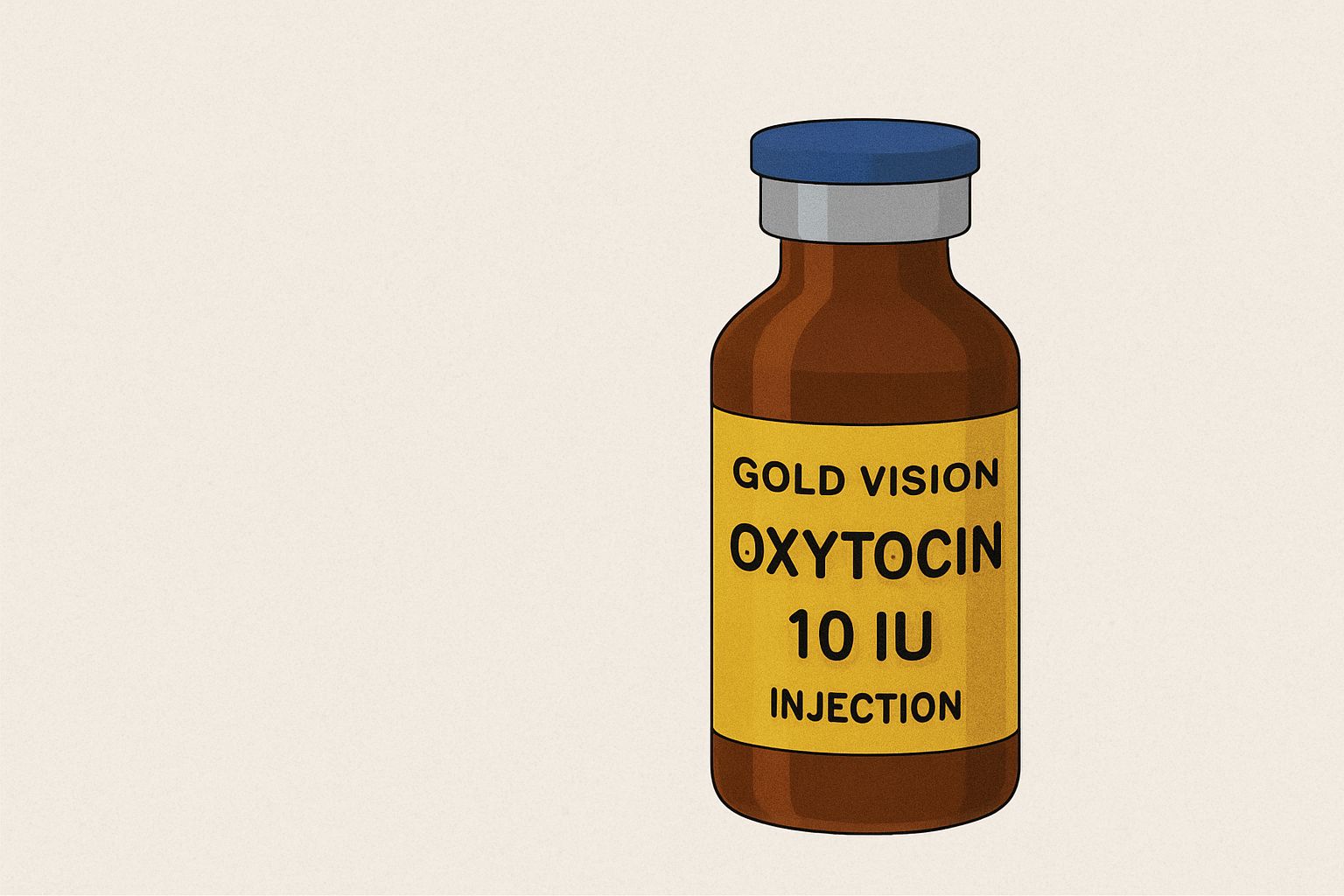
The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted Nigerians about an unregistered Gold Vision Oxytocin injection 10IU bearing a fake NAFDAC registration number. According to the agency’s website, the alert (numbered 028/2025) confirms that the product, purportedly manufactured by Anhui Hongye Pharmaceutical Co., Ltd. in China and marketed by Gold Vision Medicals in Enugu, is not officially registered.
The product was discovered during a risk-based sampling survey conducted by officers of NAFDAC’s Post-Marketing Surveillance (PMS) directorate. According to NAFDAC, further investigations revealed that three other falsified products—A-tocin injection, Extocin injection, and Claxitodin injection—were also identified in 2023, all carrying the same fake registration number, A4-9566.
The agency emphasised that these products are confirmed falsified because they do not appear in NAFDAC’s registered products database. Oxytocin, a naturally occurring hormone and pharmaceutical drug, is primarily used to induce or strengthen labour, control postpartum bleeding, and support lactation. Using unregistered or falsified oxytocin injections poses serious health risks, including ineffective uterine contractions, postpartum haemorrhage, and even maternal death.
What to Watch Out For
According to NAFDAC, consumers, healthcare professionals, and suppliers should:
-
Ensure all medicinal products are obtained from authorised or licensed suppliers.
-
Check the authenticity and physical condition of Oxytocin injections carefully.
-
Be alert for products bearing the NAFDAC registration number A4-9566, which has been confirmed as falsified.
-
Report any suspicion of substandard or falsified medicines to the nearest NAFDAC office or via the E-reporting platform on the NAFDAC website.
-
Healthcare professionals should also report any adverse reactions or side effects related to medicinal products or devices.
All NAFDAC zonal directors and state coordinators have been instructed to conduct surveillance and retrieve any of these falsified products if found. According to the agency, vigilance across the supply chain, from importers to retailers to consumers, is essential to prevent the use of unregistered or falsified Oxytocin injections.
For more information, you can view the alert directly on NAFDAC’s official website.